ZSM-5 is one of the most important zeolites used in modern chemical and petrochemical industries. Its success does not come from chance. Instead, it comes from a highly ordered and intelligent crystal structure that controls how molecules move, react, and transform. Because of this, engineers and chemists often describe ZSM-5 as a shape-selective zeolite rather than a simple adsorbent.
This article explains the ZSM-5 zeolite structure in clear terms. It covers how the framework forms, how its pore system works, why it remains stable under harsh conditions, and how these features translate into real industrial value.
What Is ZSM-5 and Why Structure Matters?
ZSM-5 is a high-silica aluminosilicate zeolite with a crystalline framework known as the MFI topology. At first glance, this may sound complex. However, the idea is simple. The material consists of a repeating, orderly arrangement of atoms that creates tiny channels throughout the crystal.
Structure matters because reactions inside zeolites happen inside these channels, not on flat surfaces. Therefore, the size, shape, and connectivity of the pores directly control which molecules can enter, react, or leave. In ZSM-5, this control remains exceptionally precise.
Because of this structural precision, ZSM-5 does not behave like a general sponge. Instead, it acts like a molecular filter and reactor combined into one solid material.
The Atomic Framework of ZSM-5 Zeolite
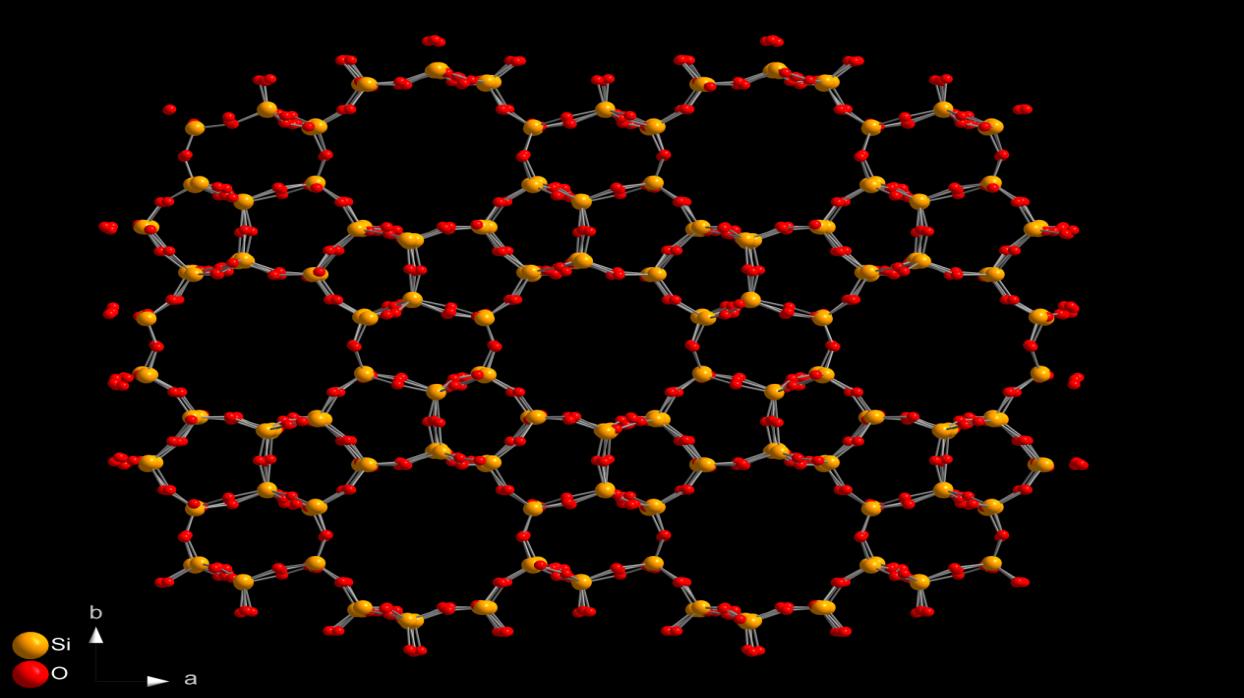
At the atomic level, ZSM-5 forms from SiO₄ and AlO₄ tetrahedra. Each tetrahedron consists of a silicon or aluminum atom surrounded by four oxygen atoms. These tetrahedra connect through shared oxygen atoms to form a rigid three-dimensional network.
When aluminum substitutes for silicon in the framework, it introduces a negative charge. This charge balances with protons or metal cations, which creates acidic sites inside the pores. These acidic sites play a major role in catalytic reactions, especially hydrocarbon conversions.
The framework is often represented in research using simplified notations rather than fixed molecular formulas. Since the structure repeats continuously, scientists describe it as an extended aluminosilicate lattice rather than a single molecule. This continuous framework explains why ZSM-5 behaves as a solid catalyst rather than a dissolvable compound.
The Dual-Channel Pore System Explained Simply
One of the most defining features of ZSM-5 is its dual-channel pore system. Unlike many zeolites that contain one dominant pore type, ZSM-5 includes two interconnected channel systems.
The first is a straight channel system that runs along one crystallographic direction. These channels provide relatively direct pathways for molecules to move through the structure.
The second is a zigzag or sinusoidal channel system that runs perpendicular to the straight channels. These channels curve slightly, which slows molecular movement and increases interaction time with active sites.
Where these channels intersect, they create confined reaction zones. Molecules entering these intersections experience steric constraints, meaning only molecules of certain shapes and sizes can react efficiently. This feature gives ZSM-5 its well-known shape selectivity.
Shape Selectivity and Molecular Control
Shape selectivity defines why ZSM-5 stands apart from many other catalysts. Instead of allowing all molecules to react freely, it selectively favors certain molecular geometries.
For example, straight-chain hydrocarbons diffuse more easily through the channels than bulky branched molecules. As a result, reactions produce specific products while limiting undesired byproducts. This control improves yield, efficiency, and product consistency.
There are three main forms of shape selectivity at work:
- Reactant selectivity, where only certain molecules enter the pores
- Product selectivity, where only certain products can exit
- Transition-state selectivity, where only specific reaction pathways fit inside the pore geometry
Together, these effects make ZSM-5 extremely valuable in complex reaction systems.
Thermal and Chemical Stability of the Structure
Another major advantage of the ZSM-5 structure is its exceptional stability. Its high silica content strengthens the framework and reduces vulnerability to steam, acids, and high temperatures.
Many industrial reactions operate above 400°C. Under these conditions, less stable materials degrade, collapse, or lose activity. ZSM-5 maintains its crystalline integrity even after long exposure to heat and pressure.
Its hydrophobic nature, which comes from a high silicon-to-aluminum ratio, also helps repel excess water. This property prevents pore blockage and extends catalyst lifetime in reactions involving hydrocarbons and limited moisture.
Because of this stability, industries can operate continuously without frequent catalyst replacement.
How Structure Influences Catalytic Performance?
The ZSM-5 structure directly controls catalytic behavior. Acidic sites inside the channels promote cracking, isomerization, and aromatization reactions. Meanwhile, the pore geometry restricts undesired secondary reactions.
For example, in hydrocarbon processing, ZSM-5 favors the formation of light olefins and aromatics while suppressing heavy coke formation. This balance improves catalyst life and product quality.
Additionally, the uniform pore size ensures consistent reaction conditions throughout the catalyst bed. As a result, performance remains predictable, which simplifies process control.
Industrial Applications Driven by Structure
ZSM-5 finds widespread use because its structure solves real industrial problems.
In petroleum refining, it plays a central role in catalytic cracking and gasoline upgrading. Its shape selectivity increases octane numbers while controlling unwanted emissions.
In petrochemical production, ZSM-5 supports processes that convert methanol into hydrocarbons. The pore system directs carbon chain growth and limits excessive polymerization.
Chemical manufacturers also use ZSM-5 for fine chemical synthesis, where selectivity determines profitability. By controlling molecular access, the zeolite improves yield and reduces purification costs.
In each case, performance links directly to structure rather than composition alone.
Tailoring the Structure for Specific Needs
Manufacturers can modify ZSM-5 properties by adjusting synthesis conditions. Changing the silica-to-alumina ratio alters acidity. Introducing metal ions adds new catalytic functions. Crystal size control influences diffusion rates.
These adjustments allow engineers to fine-tune ZSM-5 without changing its fundamental framework. As a result, the same structural concept adapts to multiple applications.
This tunability explains why ZSM-5 remains relevant even as industrial needs evolve.
Future Importance of the ZSM-5 Structure
Looking ahead, the ZSM-5 structure will continue to support advanced chemical processes. As industries seek higher efficiency and lower emissions, catalysts must deliver precision rather than brute force.
ZSM-5 aligns with this direction because it controls reactions at the molecular level. Its ability to guide molecules through confined pathways supports cleaner reactions and reduced waste.
Ongoing research focuses on combining ZSM-5 with other nanostructures to enhance performance further. These hybrid systems aim to preserve structural advantages while adding new functionalities.
Final Words
The ZSM-5 zeolite structure represents a perfect balance between order, stability, and functionality. Its interconnected channel system, strong aluminosilicate framework, and shape-selective behavior make it one of the most valuable materials in industrial catalysis.
Rather than acting as a passive support, ZSM-5 actively directs molecular behavior. This control improves efficiency, selectivity, and durability across a wide range of applications.
In advanced manufacturing and chemical processing, success increasingly depends on precision. ZSM-5 delivers that precision through structure, not complexity. That is why it remains a cornerstone material today and will continue to shape industrial innovation in the future.